![[Alternativtext]](https://www.deflagyn.com/wp-content/uploads/2022/09/hourglass.gif)
Most women don’t want to wait! They get active during this waiting time in order to contribute to the improvement of the findings.
Get active with DeflaGyn® vaginal gel
DeflaGyn® vaginal gel promotes spontaneous remission and regression of Unclear Cervical Smears or HPV‑induced‑ or p16/Ki‑67 positive Cervical Lesions or Cervical Erosions.
Take action like many women all over the world. Women who take charge of situations. So don’t put it off any longer – get active!

Days of Therapy
Countries
Partners

DeflaGyn® Application Set
The vaginal gel is available as part of the DeflaGyn® Application Set, a medical device system that includes the gel in a bottle and applicators for dosage and comfortable administration.
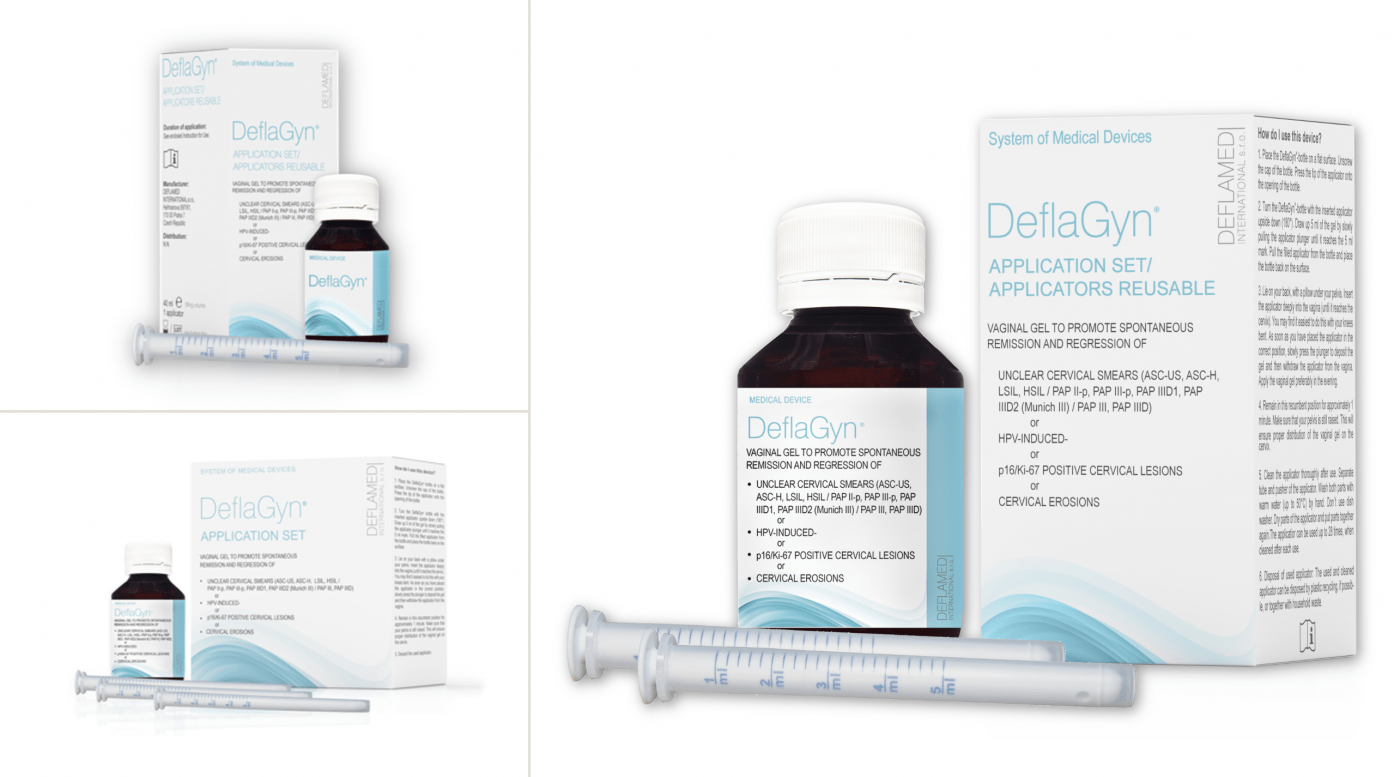
Available Sets
- DeflaGyn® 150ml / 2 reusable applicators – 28 days therapy / one cycle
- DeflaGyn® 40ml / 1 reusable applicator – 7 days therapy (travel pack)
- DeflaGyn® 150ml / 28 single use applicators – 28 days therapy / one cycle (will be discontinued)
Active Ingredients
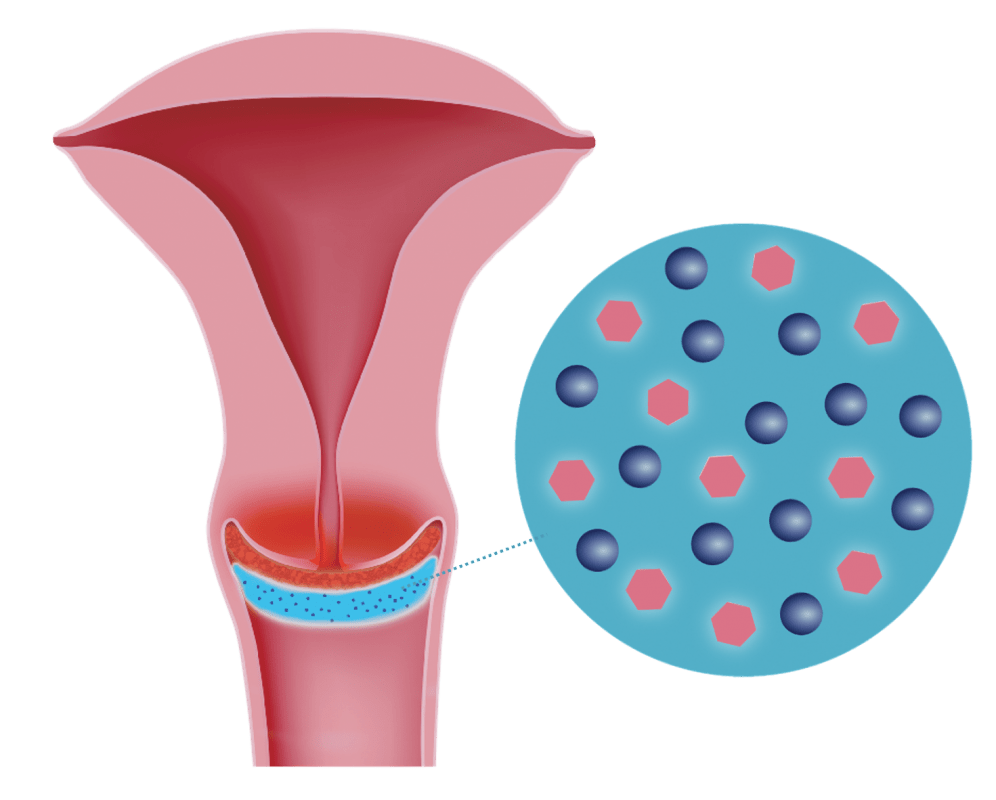
Highly dispersed micronised Silicon Dioxide (SiO2)
Antioxidative DEFLAMIN® (biologically activated Selenium)
DeflaGyn® vaginal gel contains highly dispersed micronized Silicon Dioxide (SiO2) particles as main ingredient and antioxidative DEFLAMIN®
(biologically activated Selenium, a patented combination of Sodium Selenite and Citric Acid).
Composition of DeflaGyn® vaginal gel
| Composition of DeflaGyn® Vaginal Gel | Quantity1 (mg/5ml) | Function of Ingredient | |
| Active ingredients | |||
| Colloidal anhydrous silica (silicon dioxide) | 10.0 | Inert adsorbent | |
| DEFLAMIN® | Citric acid | 24.8 | pH adjuster and antioxidant enhancer |
| Sodium selenite pentahydrate2 | 0.83 | Antioxidant | |
| Adjunctive agents | |||
| Water | 4899.7 | Solvent | |
| Hydroxyethyl cellulose | 99.24 | Gelling agent | |
| Preservatives | |||
| Potassium sorbate | 5.0 | Preservative | |
| Sodium benzoate | 2.5 | Preservative | |
1single application
2selenium equivalent 0.25 mg
Three-step mechanism of action
1. Adsorption Effect
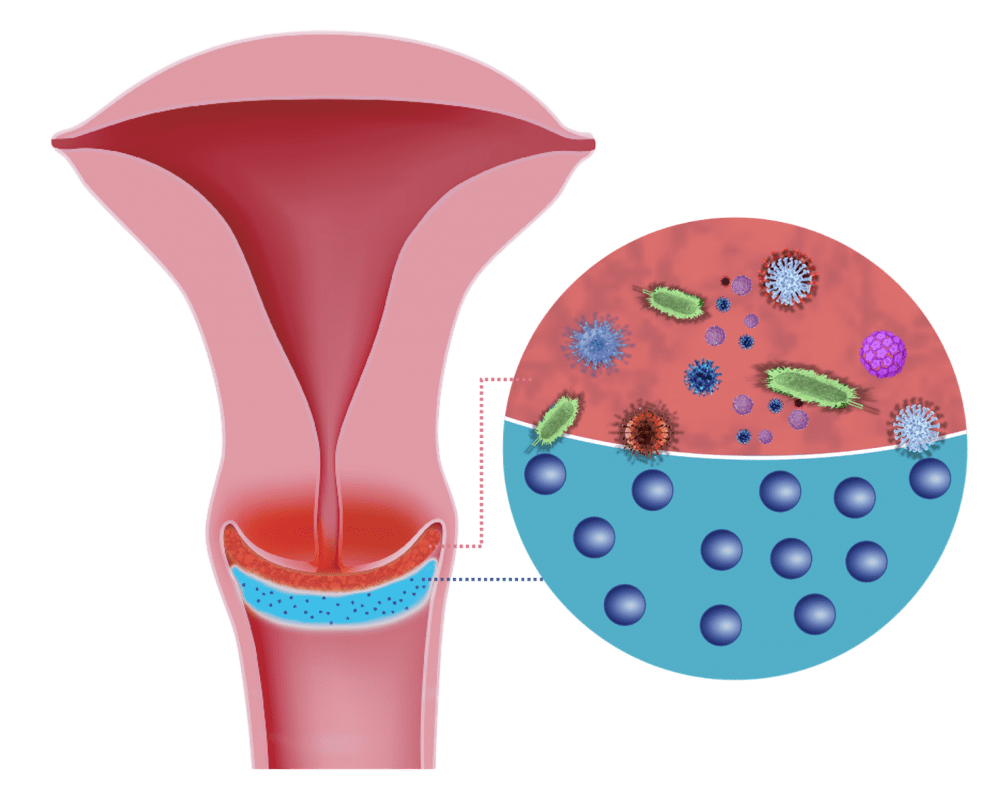
Highly dispersed, micronised silicon dioxide particles adsorb pathogens (bacteria, viruses, fungi, cell residues, irritating particles) from the surface of the cervix
The „adsorbing effect“ of micronised silicon dioxide has been verified and photographically recorded through fluorescence microscopic tests by Zeta
Partikel Analytik GmbH. The tests, conducted with Staphylococcus aureus, are documented extensively.
2. Binding Effect
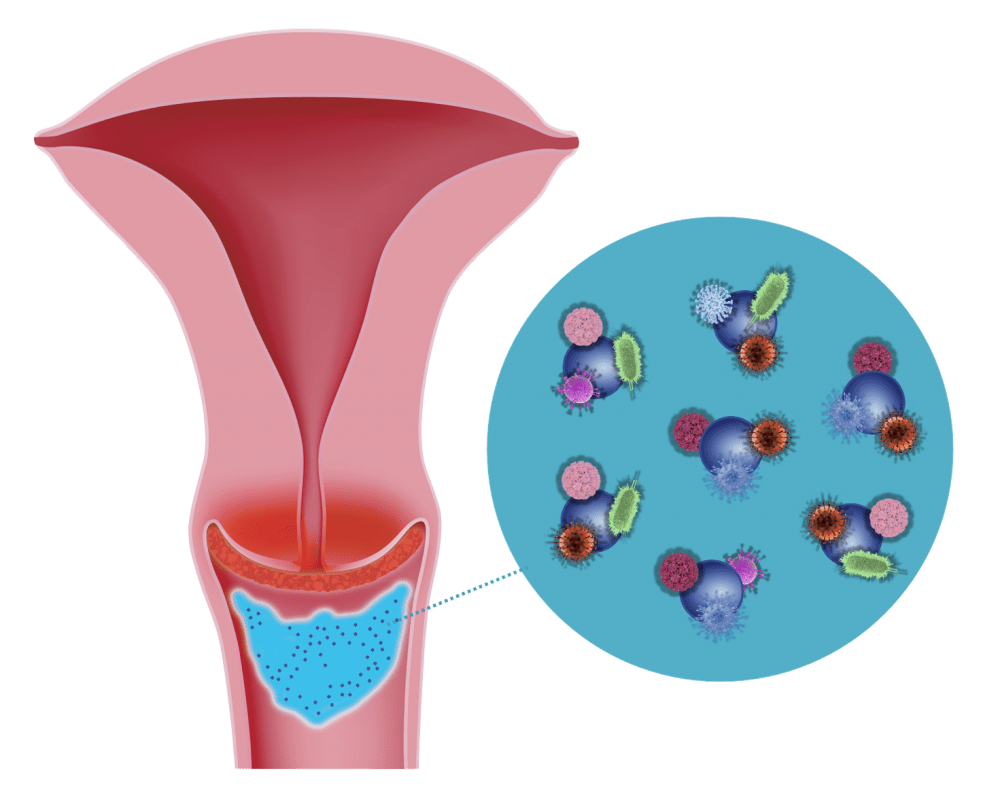
Silicon dioxide binds these pathogens and thus prevents them from spreading on the surface of the cervix and in the vagina
The „binding effect“ of micronised silicon dioxide has been verified and quantified through in-vitro tests using a vaginal secretion model test system by Prof. Geoffrey Lee / Friedrich-Alexander University of Erlangen.
The tests, conducted with Bovine Serum Albumin (BSA), are described in detail.
3. Antioxidative Effect
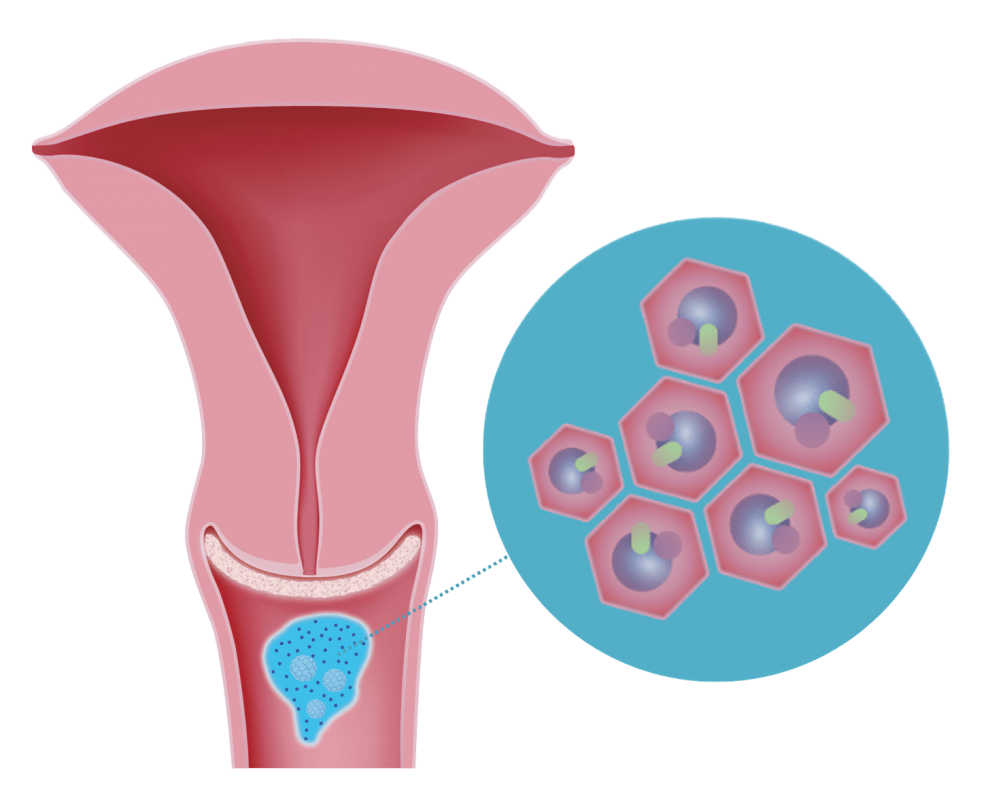
The adsorbed and bound pathogens are neutralised by the antioxidant properties of DEFLAMIN®
Once they have been bound, the potentially pro-oxidant influence of the adsorbed pathogens is neutralised by the antioxidant properties of DEFLAMIN® the patented combination of sodium selenite and citric acid.
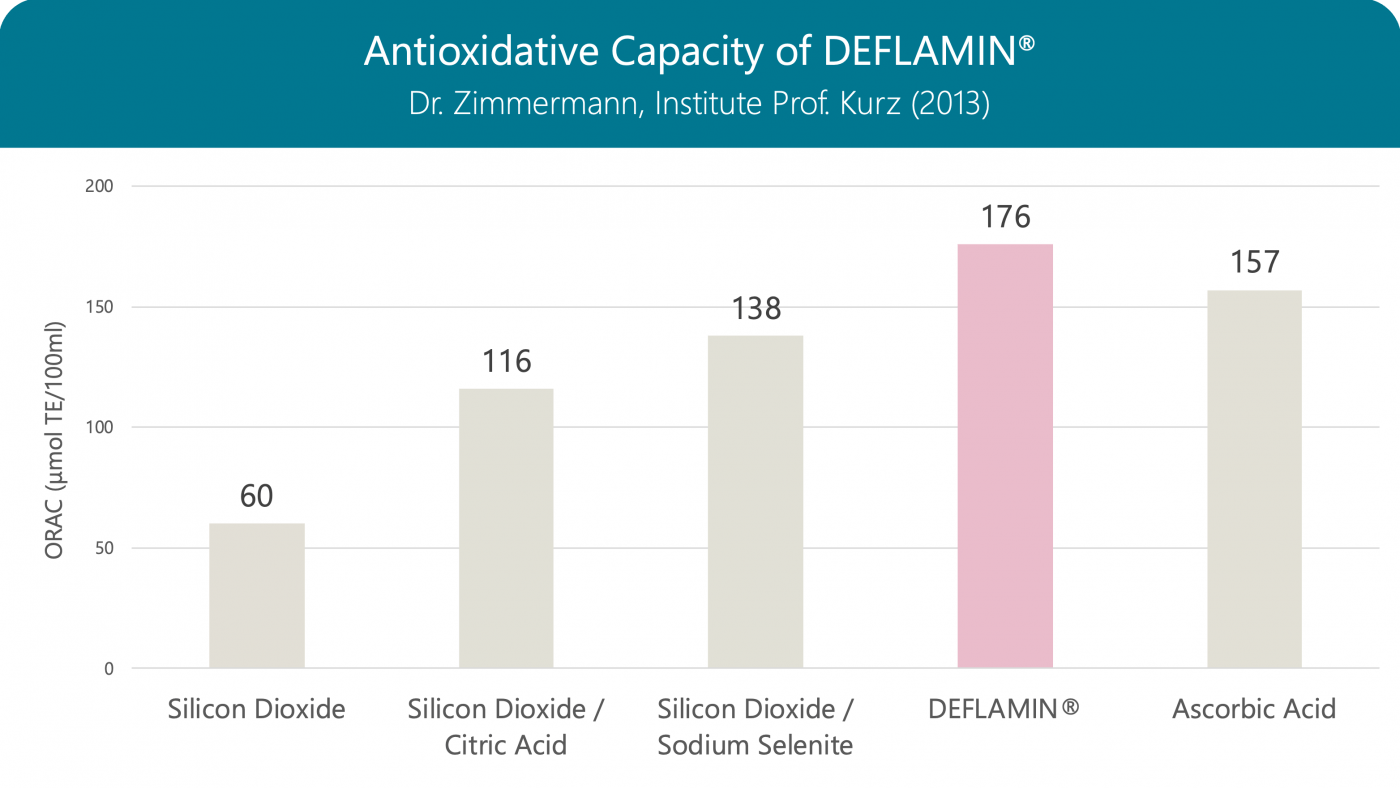
Antioxidative Capacity
The antioxidative capacity of DEFLAMIN® (the combination of citric acid and sodium selenite) is stronger than that of the individual components, and even stronger than that of vitamin C.
Moreover, citric acid lowers / stabilizes the pH and thus the acid vaginal milieu. However, this is only a secondary effect. A lowering of the vaginal pH alone would not be sufficient to fight the pathogens because there are many acid-resistant bacteria and viruses, in particular the HP virus.
Oxygen Radical Absorbance Capacity (ORAC): When measuring, the vitamin E derivative Trolox serves as a reference, so the result is given in Trolox equivalents (TE)
Clinically Proven Therapeutic Efficacy
How it works
Due to the described three-step mechanism of action the irritated cervical epithelium is relieved, which improves the condition for a spontaneous remission.
The adsorbed, bound and neutralized pathogens are eliminated with the excretion of the vaginal gel.
Why it's so effective
In conformity with the guidelines the „watchful waiting“ period can be bridged by using DeflaGyn® vaginal gel for a period of 3 x 28 days.
DeflaGyn® vaginal gel relieves the irritated cervical epithelium and the vaginal mucosa of potentially irritating pathogens.
DeflaGyn® vaginal gel thus promotes spontaneous remission by adjusting the milieu of the cervical and vaginal secretion during the psychologically stressful period of „watchful waiting“.

Improved Cytological Outcome
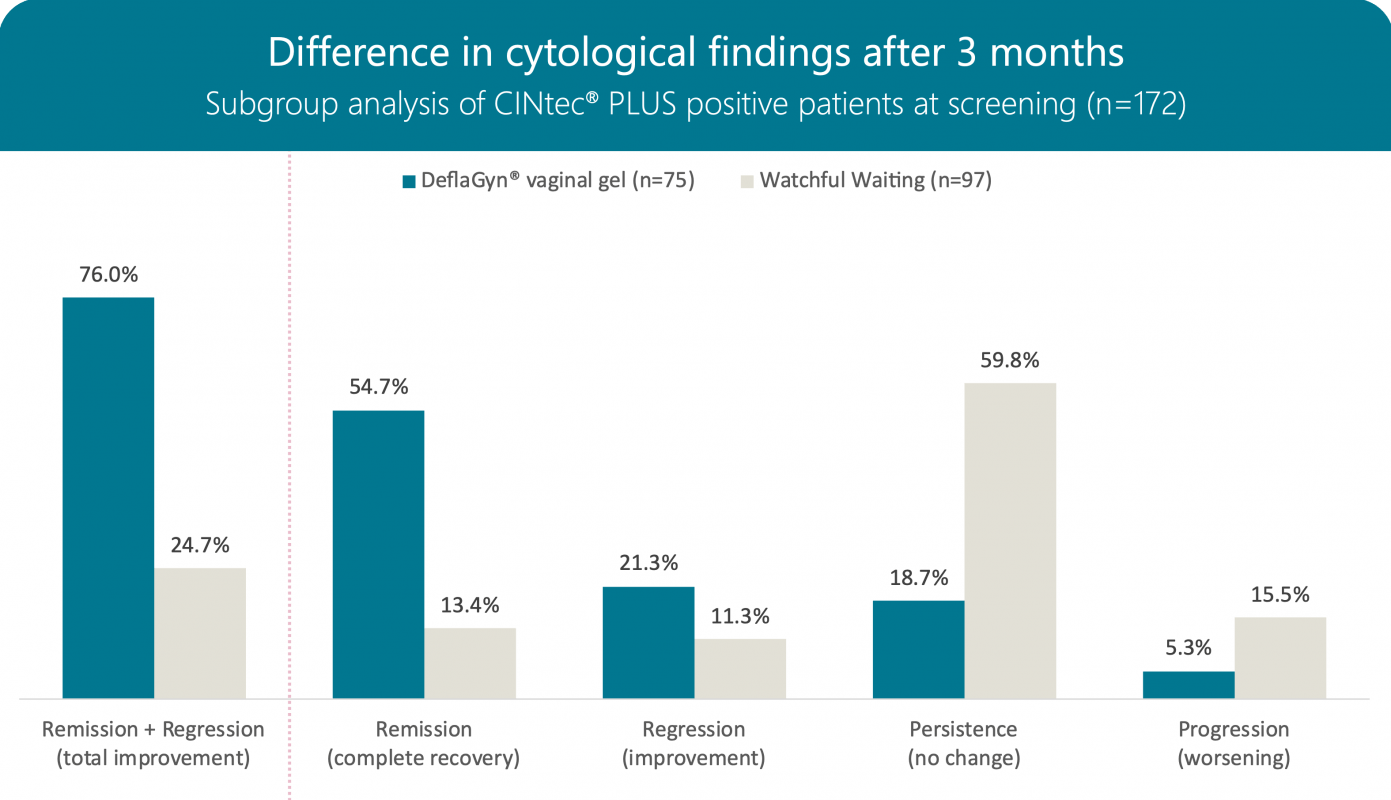
Cytology after 3 months
172 women with a histological diagnosis of CIN2 or p16‑positive CIN1 lesions were selected based on a positive cytological p16/Ki‑67 test.
For 3 months, 75 patients in the active arm daily administered 5ml of DeflaGyn® vaginal gel. 97 patients in the control arm underwent no treatment („watchful waiting“).
At 3 months, cytological remission and regression was observed in 76% (57/75) of patients in the active arm compared with 25% (24/97) in the control arm. Progression occurred in 5% (4/75) of the active arm compared with 15% (15/97) of the control arm.
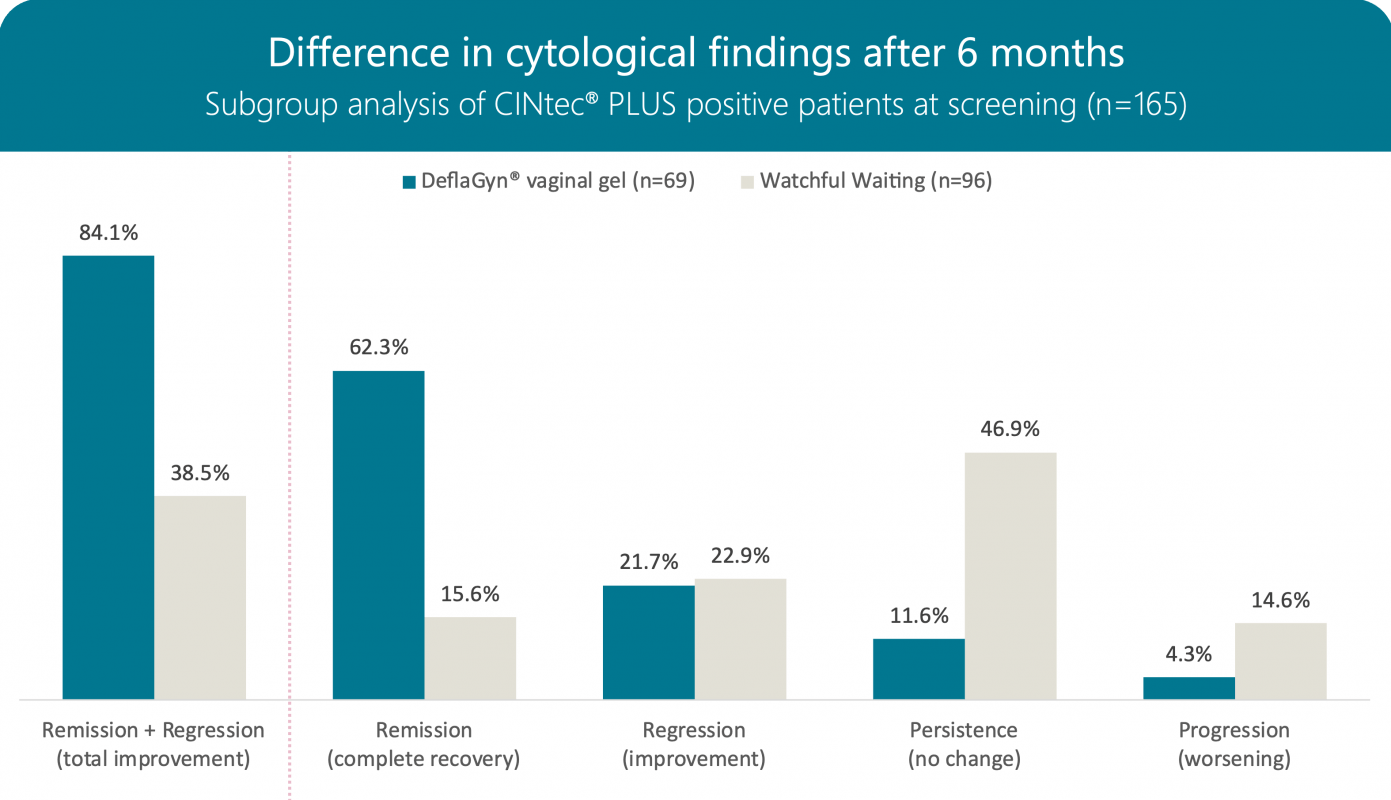
Cytology after 6 months
After a further observation period without treatment in the active arm a similar statistically significant difference was still present at 6 months, which was mainly due to the disappearance of low‑grade cytological findings (ASC‑US and LSIL). 58/69 patients (84%) in the active arm and 37/96 patients (39%) in the control arm showed regression or remission.
DeflaGyn® vaginal gel is effective for enhancing the remission and regression of cervical lesions and preventing their progression.
Major AL, Skřivánek A, Grandjean EM, Dvořák V, Malík T, Pluta M and Mayboroda I (2021) An Adsorptive and Antioxidant Vaginal Gel Clears High-Risk HPV- and p16/Ki-67-Associated Abnormal Cytological Cervical Findings: A post-hoc Subgroup Analysis of a Prospective Randomized Controlled Trial on CIN2 and p16 Positive CIN1. Front. Med. 8:645559. doi: 10.3389/fmed.2021.645559
Clearing High‑Risk HPV
HPV is the most common viral infection of the reproductive tract. Most sexually active women and men will be infected at some point in their lives, and some may be repeatedly infected. More than 90% of the infected populations eventually clear the infection.
Although most HPV infections clear up on their own and most pre-cancerous lesions resolve spontaneously, there is a risk for all women that HPV infection may become chronic and pre-cancerous lesions progress to invasive cervical cancer.
It takes 15 to 20 years for cervical cancer to develop in women with normal immune systems. It can take only 5 to 10 years in women with weakened immune systems, such as those with untreated HIV infection (WHO).
The effect of DeflaGyn® vaginal gel on high‑risk HPV
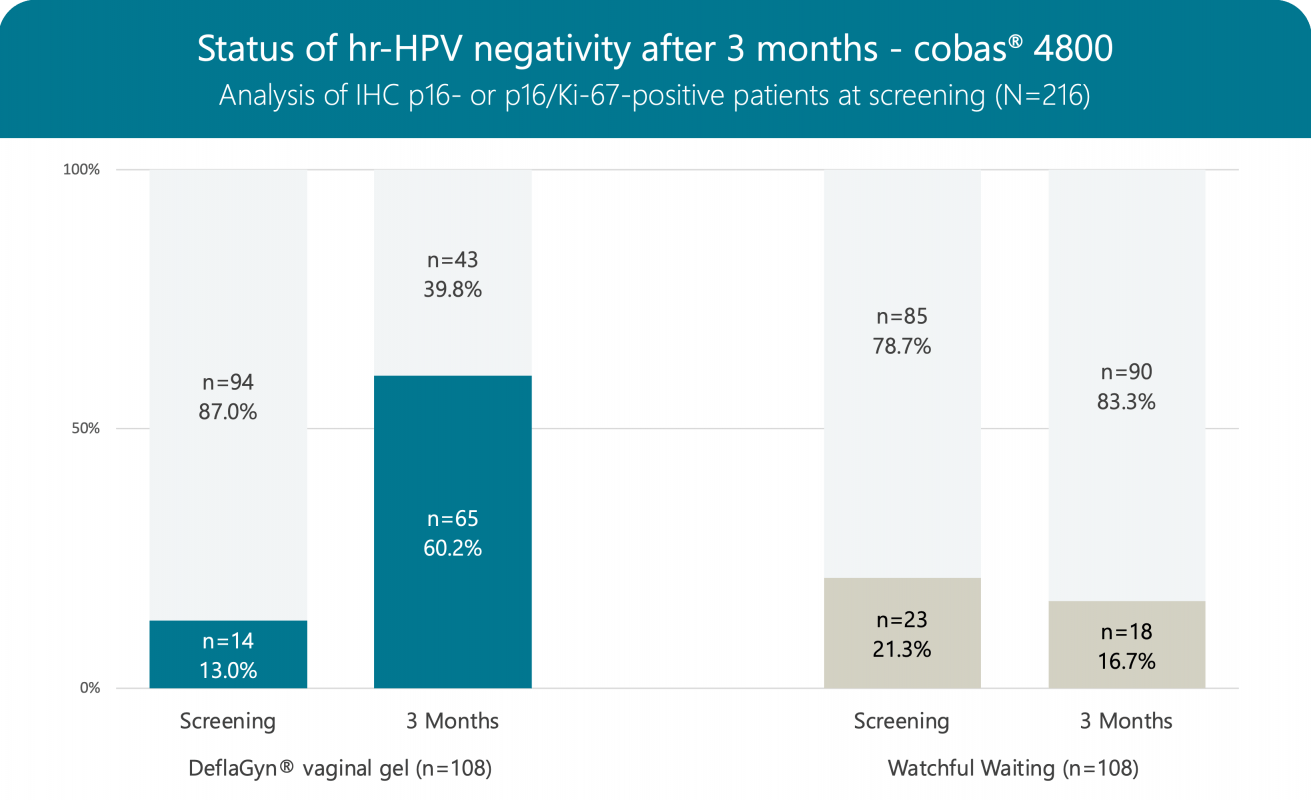
The hr‑HPV clearance (positive at screening vs. negative after 3 months) is 54.3% in patients treated with DeflaGyn® vaginal gel vs. only 10.6% in non-treated patients following the strategy of „Watchful Waiting“.
Out of 14 patients being negative for hr‑HPV testing at screening in the treated group, all 14 patients remained negative after 3 months, whereas out of 23 patients negative at screening in the non-treated group 14 patients (60.9%) were tested positive after 3 months.
DeflaGyn® vaginal gel binds, neutralizes and eliminates hr‑HPV hence supporting the cervical and vaginal epithelium to recover and to regenerate physiologically.
DeflaGyn® vaginal gel is a potential therapy regimen for patients with HPV infected cervical lesions.
hr-HPV after 3 months
| High-Risk HPV status at screening | High-Risk HPV status after 3 months | ||
|---|---|---|---|
| cobas® 4800 | Patients n (%) | Positive | Negative |
| DeflaGyn® vaginal gel – Active Arm1 | |||
| Positive | 94 (87.0) | 43 (45.7) | 51 (54.3) |
| Negative | 14 (13.0) | 0 (0.0) | 14 (100.0) |
| Total | 108 (100.0) | 43 (39.8) | 65 (60.2) |
| Watchful Waiting – Control Arm2 | |||
| Positive | 85 (78.7) | 76 (89.4) | 9 (10.6) |
| Negative | 23 (21.3) | 14 (60.9) | 9 (39.1) |
| Total | 108 (100.0) | 90 (83.3) | 18 (16.7) |
1Active Arm 3 months treatment with DeflaGyn® vaginal gel
2Control Arm 3 months Watchful Waiting (no treatment)
Reduced Oncogenic Risk
The CINtec® PLUS Cytology test detects the simultaneous presence of the two biomarkers p16 and Ki-67 (dual-stain). This abnormality is associated with HPV infections that are oncogenically transforming and can, if left untreated, progress to pre-cancer or cancer.
The molecular mechanism for detecting p16 overexpression is independent of the high-risk HPV type. A positive result of these two biomarkers in a
single cell indicates that a woman is significantly higher at risk for cervical disease.
Women with negative dual-stain results are at significantly lower risk for cervical disease and their bodies can be given more time to clear the HPV infection on their own. This may reduce the number and frequency of follow-up visits, saving many patients worry and time.
The effect of DeflaGyn® vaginal gel on oncogenically transforming HPV‑infections
DeflaGyn® vaginal gel adsorbs, binds, neutralises and eliminates infectious particles from the vaginal secretion and the cervical mucosa. This biophysical mechanism of the vaginal gel enables the epithelium to regenerate physiologically and thus supports spontaneous remission.
With DeflaGyn® vaginal gel – consequently reiterated daily over a 3 months treatment period – a reinfection of the epithelium with infectious particles is minimized and in many cases even ceased.
DeflaGyn® vaginal gel can reverse the oncogenic activity of hr‑HPV by altering the vaginal milieu within three months of treatment.
p16/Ki-67 after 3 months treatment with DeflaGyn® vaginal gel
| CINtec® PLUS status at screening | CINtec® PLUS status after 3 months | |||
|---|---|---|---|---|
| CINtec® PLUS | Patients n (%) | Missing | Negative | Positive |
| DeflaGyn® vaginal gel – Active Arm1 | ||||
| Negative | 31 (28.7) | 1 (3.2) | 30 (96.8) | 0 (0.0) |
| Positive | 77 (71.3) | 5 (6.5) | 59 (76.6) | 13 (16.9) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 108 (100.0) | 6 (5.6) | 89 (82.4) | 13 (12.0) |
| Watchful Waiting – Control Arm2 | ||||
| Negative | 8 (7.4) | 0 (0.0) | 6 (75.0) | 2 (25.0) |
| Positive | 99 (91.7) | 1 (1.0) | 20 (20.2) | 78 (78.8) |
| Missing | 1 (0.9) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
| Total | 108 (100.0) | 1 (0.9) | 26 (24.1) | 81 (75.0) |
1Active Arm 3 months treatment with DeflaGyn® vaginal gel
2Control Arm 3 months Watchful Waiting (no treatment)
p16/Ki-67 after 6 months
| CINtec® PLUS status at screening | CINtec® PLUS status after 6 months | |||
|---|---|---|---|---|
| CINtec® PLUS | Patients n (%) | Missing | Negative | Positive |
| DeflaGyn® vaginal gel – Active Arm1 | ||||
| Negative | 30 (29.4) | 2 (6.7) | 28 (93.3) | 0 (0.0) |
| Positive | 72 (70.6) | 6 (8.3) | 60 (83.3) | 6 (8.3) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 102 (100.0) | 8 (7.8) | 88 (86.3) | 6 (5.9) |
| Watchful Waiting – Control Arm2 | ||||
| Negative | 8 (7.5) | 0 (0.0) | 5 (62.5) | 3 (37.5) |
| Positive | 98 (91.6) | 1 (1.0) | 21 (21.4) | 76 (77.6) |
| Missing | 1 (0.9) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
| Total | 107 (100.0) | 1 (0.9) | 26 (24.3) | 80 (74.8) |
1Active Arm 3 months treatment with DeflaGyn® vaginal gel + 3 months observation period without treatment
2Control Arm 6 months Watchful Waiting (no treatment)
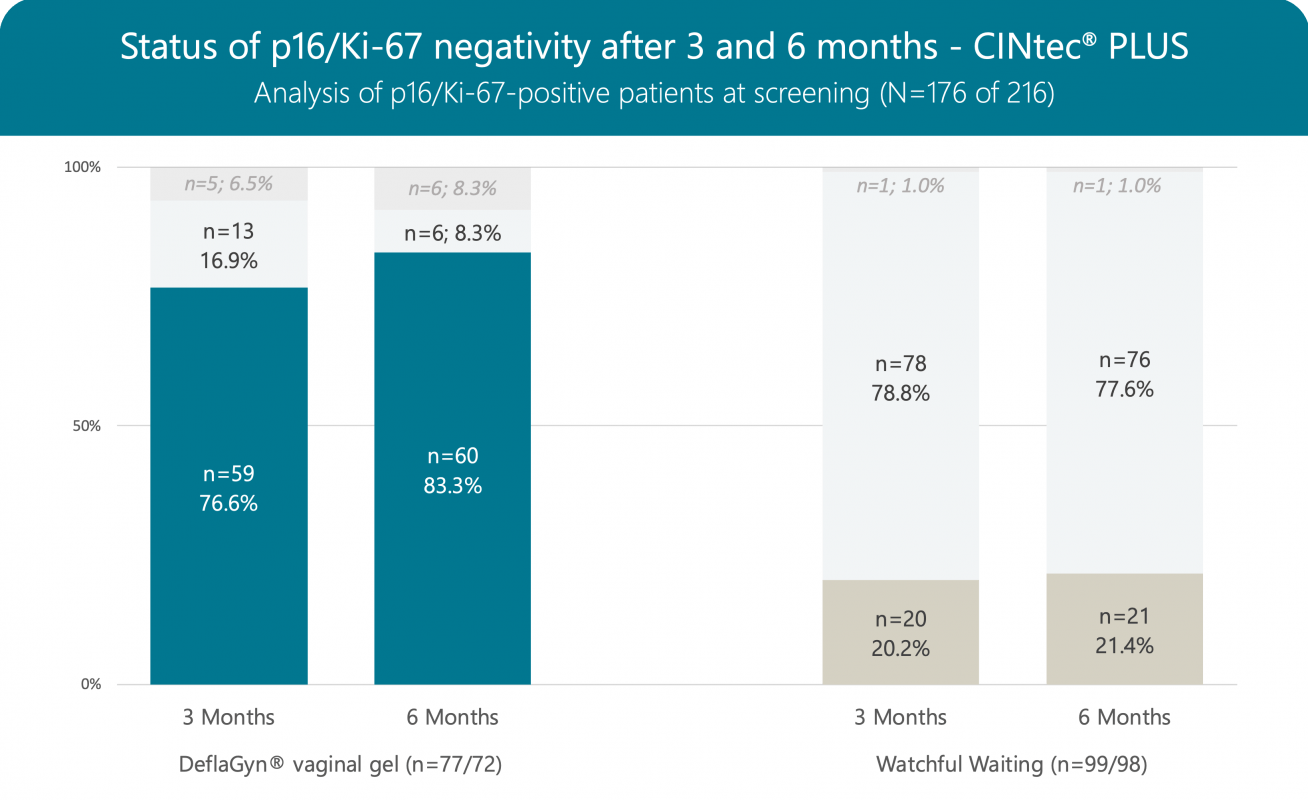
CINtec® PLUS Summary
The p16/Ki‑67 clearance (positive at screening vs. negative after 3 months) is 76.6% in patients treated with DeflaGyn® vaginal gel vs. only 20.2% in non-treated patients.
Out of 31 patients testing negative for CINtec® PLUS at screening in the treated group 30 patients (96.8%) remained negative after 3 months and no patient tested positive (0.0%), whereas 2 out of 8 patients (25.0%) were tested positive in non-treated patients.
After 6 months (3 months treatment with DeflaGyn® vaginal gel plus 3 months observation period without treatment) negativity for p16/Ki-67 increased from 76.6% to 83.3% in the active arm. In patients following the strategy of watchful waiting over 6 months negativity increased from 20.2.% to 21.4% only.
Out of 30 patients testing negative for CINtec® PLUS at screening in the treated group 28 patients (93.3%) remained negative after 6 months and still no patient tested positive (0.0%). In the non-treated group 3 out of 8 patients (37.5%) were tested positive.
DeflaGyn® vaginal gel is influencing the oncogenic progress substantially. The significant reduction of oncogenic risk factors (83.3% p16/Ki‑67 negativity) indicates that DeflaGyn® vaginal gel is a potential therapy regimen for patients with HPV‑infected cervical lesions.
DeflaGyn® vaginal gel -
significant reduction of oncogenic risk factors
Physiological regeneration of the epithelium
With the described three‑step mechanism of action DeflaGyn® vaginal gel blocks the HPV‑reinfection and HPV‑replication which improves the condition for a spontaneous remission.
p16/Ki‑67 negativity by HPV‑clearance
Starving out the reinfection process through consequent HPV‑elimination
Application of DeflaGyn® vaginal gel
EFFECTIVE

SAFE

EASY
To achieve the best possible effect, apply DeflaGyn® vaginal gel once a day for a period of 3 x 28 days. Best practice is to use DeflaGyn® vaginal gel in the evening, before going to bed.
Non-menstruating patients
DeflaGyn® vaginal gel should be used for a duration of 3 x 28 days. After 28 days of treatment, please stop using DeflaGyn® vaginal gel for 3 days. Continue treatment for another 28 days and stop for another 3 days. Afterwards continue with the third treatment cycle.
Menstruating patients
Do not use DeflaGyn® vaginal gel while you are menstruating (approx. 3‑5 days/cycle). It is not necessary to stop treatment for another 3 days, as menstruation already counts as a treatment break. You can start using DeflaGyn® vaginal gel on any day of your cycle.
How to use the applicator
Please use the applicator in combination with DeflaGyn® vaginal gel to ensure the correct application and dosing of the vaginal gel.
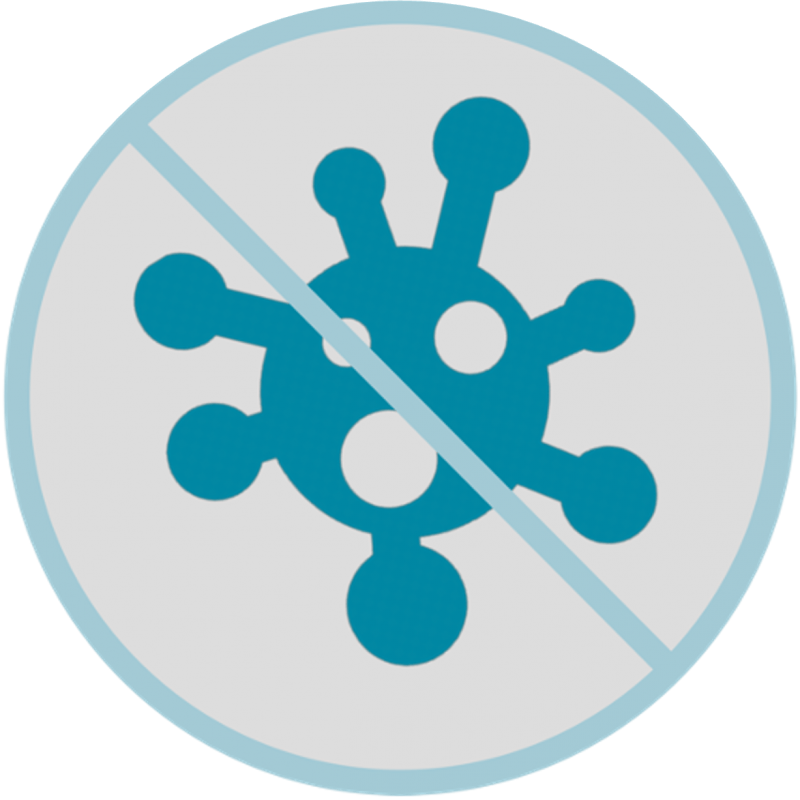
Shake bottle well before use. Draw up 5 ml of the gel by slowly pulling the applicator plunger until it reaches the 5 ml mark and applicate the gel deeply into the vagina (until it reaches the cervix).
1 - fit the tip

Place the DeflaGyn® vaginal gel bottle on a flat surface. Unscrew the cap of the bottle. Press the tip of the applicator onto the opening of the bottle.
2 - flip & pull
Turn the DeflaGyn® vaginal gel bottle with the inserted applicator upside down (180°). Draw up 5 ml of the gel by slowly pulling the applicator plunger until it reaches the 5 ml mark. Pull the filled applicator from the bottle and place the bottle back on the surface.
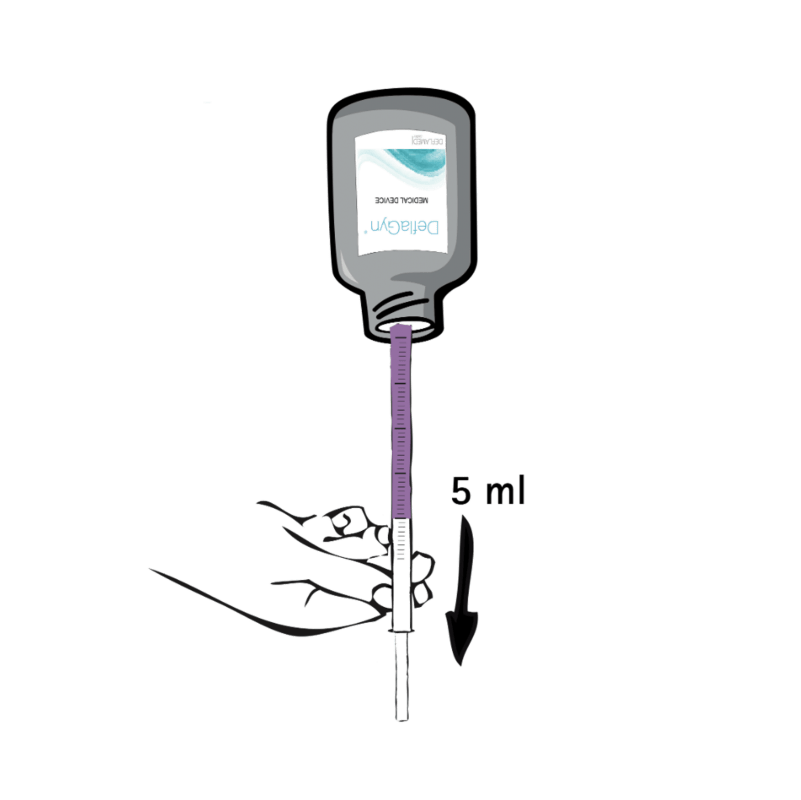
3 - insert & push
Lie on your back, with a pillow under your pelvis. Insert the applicator deeply into the vagina (until it reaches the cervix). You may find it easiest to do this with your knees bent. As soon as you have placed the applicator in the correct position, slowly press the plunger to deposit the gel.
Apply the vaginal gel preferably in the evening.
4 - pull out & rest
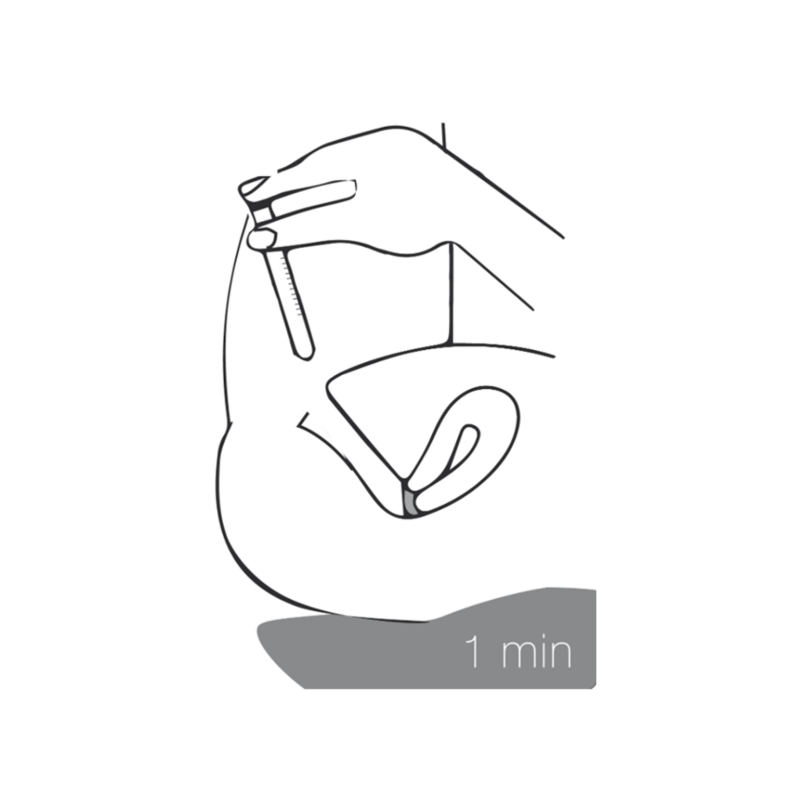
Withdraw the applicator from the vagina and remain in this recumbent position for approximately 1 minute. Make sure that your pelvis is still raised. This will ensure proper distribution of the vaginal gel on the cervix.
5 - clean & dry
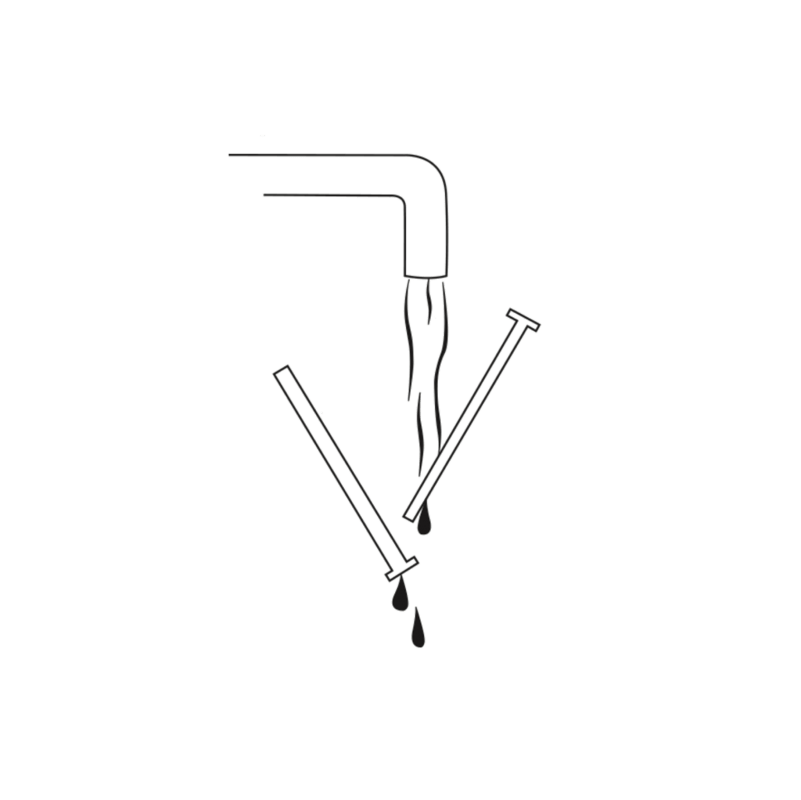
Clean the applicator thoroughly after use. Separate tube and pusher of the applicator. Wash both parts with warm water (up to 50°C) by hand. Don’t use dish washer. Dry parts of the applicator and put parts together again.
The applicator can be used up to 28 times, when cleaned after each use.
Disposal
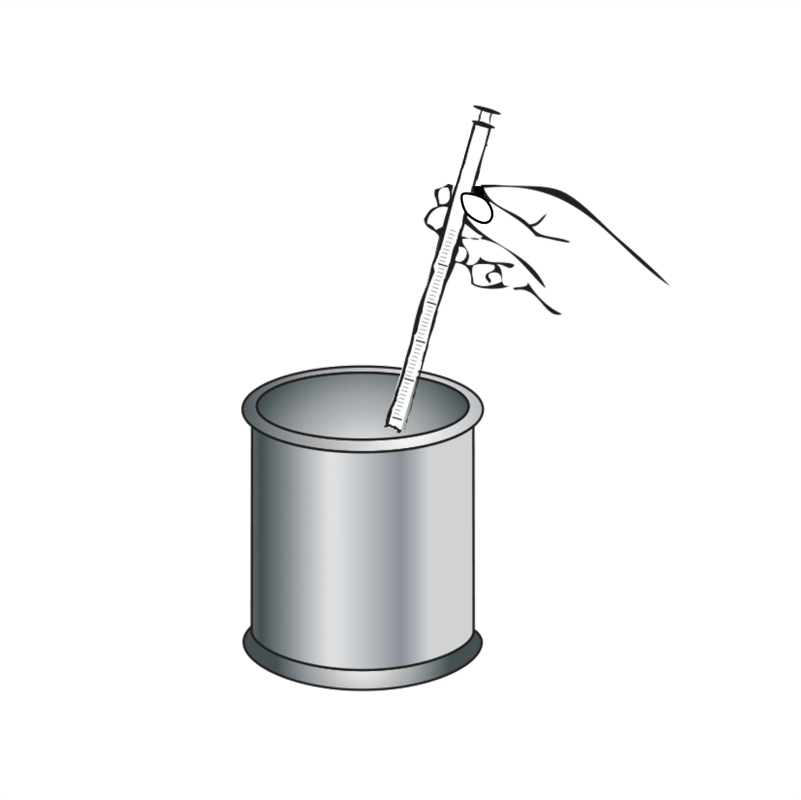
The used and cleaned applicator can be disposed by plastic recycling, if possible, or together with household waste.
Note
After application, it is possible that some of the inserted gel may leak out. This gel may appear slightly reddish and can discolor your underwear.
However, the discoloration will disappear after washing. You can also use a sanitary pad.
Do not use the applicator if the cleaning was not done thoroughly and in case the applicator is damaged. Use the applicator only for DeflaGyn® vaginal gel.
Reusable Applicators
- Easy to use and to handle
- Improved sliding mechanism
- Reusable up to 28 times
- Sustainable due to less packing material
- Environmentally friendly – less plastic