Pap tests and human papillomavirus (HPV)
Cervical screening makes use of cytology to identify women at increased risk of cervical pathology. It is an essential part of a woman’s routine health care.
Nearly all cases of cervical cancer are caused by infection with sexually transmitted oncogenic or high‑risk types of human papillomavirus (hr‑HPV).
Pap Test Results

A Pap test result can be normal, unclear, or abnormal. A normal result means that no cell changes were found on your cervix, but it is common for test results to come back unclear.
An abnormal result means that cell changes were found on your cervix. Abnormal changes on your cervix are likely caused by HPV.
Pap test results usually come back from the lab in about 1-3 weeks. If you don’t hear from your health care provider, call and ask for your test results.
Make sure you receive your test results and understand any follow-up visits or treatments that you need. Always follow your health care provider’s recommendations.
Watchful Waiting
The waiting periods recommended in many national guidelines after an unclear or abnormal Pap smear mean a treatment-free interval of several months, which is an anxious period for those concerned.
This phase of uncertainty, observation and monitoring without therapeutic measures is also called „watchful waiting“.
Studies have shown that patients who are informed of a conspicuous Pap cytology test result generally react with anxiety, panic and stress.
These reactions occur regardless of the severity of the findings and can be traced back to a lack of information about causes, prevention, importance of the findings and treatment options.

Keep in Mind
- It is common for cytological test results to come back unclear.
- Many unclear or abnormal findings may improve on their own – also described as spontaneous remission and regression – which justifies the watchful waiting strategy recommended in many national guidelines, but
- Publications consistently show that not only remission but also progression rates increase over time. The rate of progression to higher-grade lesions is reported as 16% over a period of 48 months.
- Abnormal changes on your cervix are likely caused by HPV.
- Even when initial cytological findings are still non-pathological, women who tested positive for HPV are at higher risk of developing pre-cancerous lesions.
- There is a risk for all women that the HPV infection may become chronic and pre-cancerous lesions progress to invasive cervical cancer, but
- An abnormal test result does not mean you have cervical cancer.
- The earlier HPV‑induced cervical lesions or oncogenically transforming HPV infections are detected and treated, the better.
- HPV infections that are oncogenically transforming can, if left untreated, progress to pre-cancer or cancer.
- Women testing negative for oncogenically transforming HPV infections are at significantly lower risk for cervical disease.
So Why Wait?
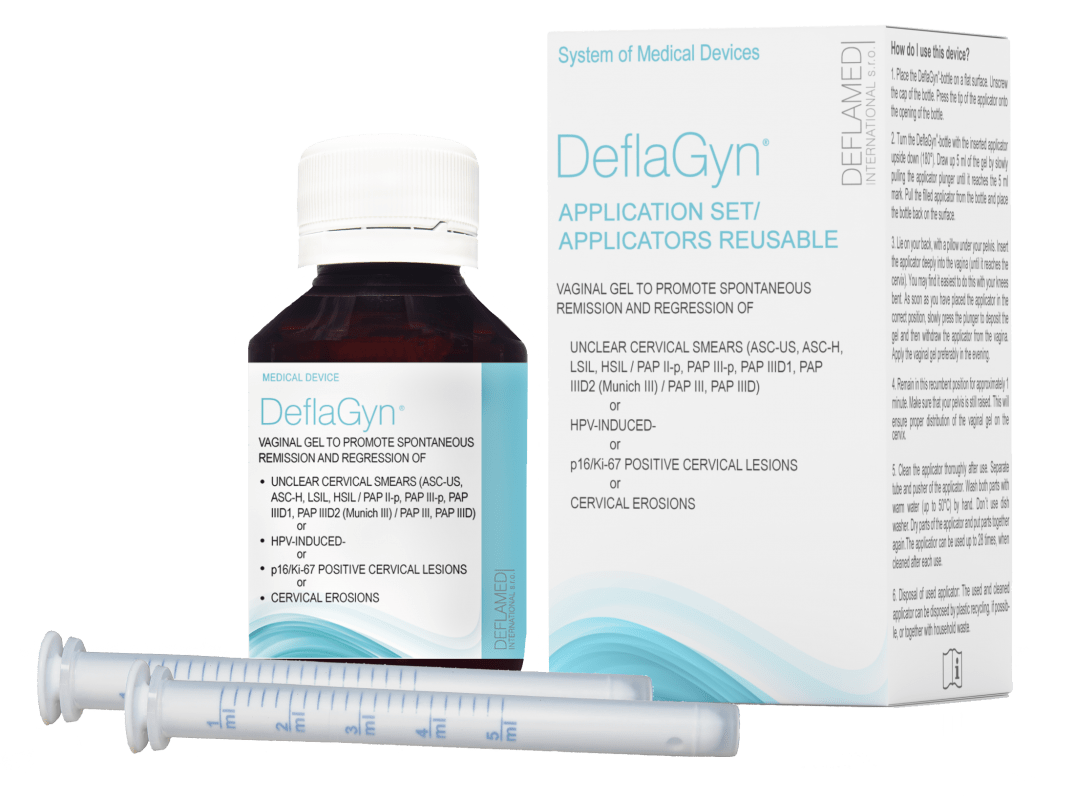
DeflaGyn® vaginal gel was specially developed to provide treatment and support during this stressful „watchful waiting“ period in conformity with the guidelines.
Most women don’t want to wait! They get active during this waiting time in order to contribute to the improvement of the findings.

DeflaGyn® vaginal gel - Certified Intended Use
DEFLAGYN® VAGINAL GEL PROMOTES SPONTANEOUS REMISSION AND REGRESSION OF
- UNCLEAR CERVICAL SMEARS
- ASC‑US, LSIL, ASC‑H, HSIL
- PAP II‑p, PAP III‑p, PAP IIID1, PAP IIID2 (Munich III)
- PAP III, PAP IIID
- HPV‑INDUCED CERVICAL LESIONS
- p16/Ki‑67 POSITIVE CERVICAL LESIONS
- CERVICAL EROSIONS
Convincing Clinical Evidence of DeflaGyn® vaginal gel
DeflaGyn® vaginal gel is an innovative Medical Device class IIa complying with Medical Device Directives 93/42/EEC and Directive 2007/47/EC.
DeflaGyn® vaginal gel is effective for enhancing the remission and regression of cervical lesions and preventing their progression.
DeflaGyn® vaginal gel is influencing oncogenic progress substantially, indicating that the vaginal gel is a potential therapy regimen for patients with HPV infected cervical lesions.
The alteration of the vaginal milieu by the DeflaGyn® vaginal gel for 3 months treatment can reverse the oncogenic activity of hr‑HPV.
DeflaGyn® vaginal gel also conforms to many national gynecological guidelines because it is used primarily in the „watchful waiting“ observation periods.
DeflaGyn® vaginal gel - Most Innovative Product 2021 and 2022
Pharma Trend is the benchmark study of innovation and sustainability in pharmaceutical companies. The effectiveness and tolerability of the products, and the benefits in treatment observed so far are the most decisive criteria in granting for the “Most Innovative Product” award.
What differentiates the “Most Innovative Product award” from other accolades in the pharmaceutical industry is its jury.
The benefits of the products are evaluated exclusively by doctors, pharmacists, and patients, based on their experience of applying the treatment.
Experts' Opinions on DeflaGyn® vaginal gel
Excellent efficacy and safety
Dr. Aleš Skřivánek, MD., PhD. – Czechia

Aleš Skřivánek
G-CENTRUM
Olomouc
Significant remission tendency during stressful „watchful waiting“
Univ. Prof. DDr. Johannes Huber – Austria

Johannes Huber
Univ. Hospital
Vienna
Extremely favourable risk-benefit profile
Univ. Prof. Dr. Doris Maria Gruber – Austria

Doris Gruber
Univ. Hospital
Vienna
Revolution in the treatment of pre-cancerous stages
Prof. DDDr. Attila Major – Switzerland

Attila Major
Femina Gyn. Center
Geneva

